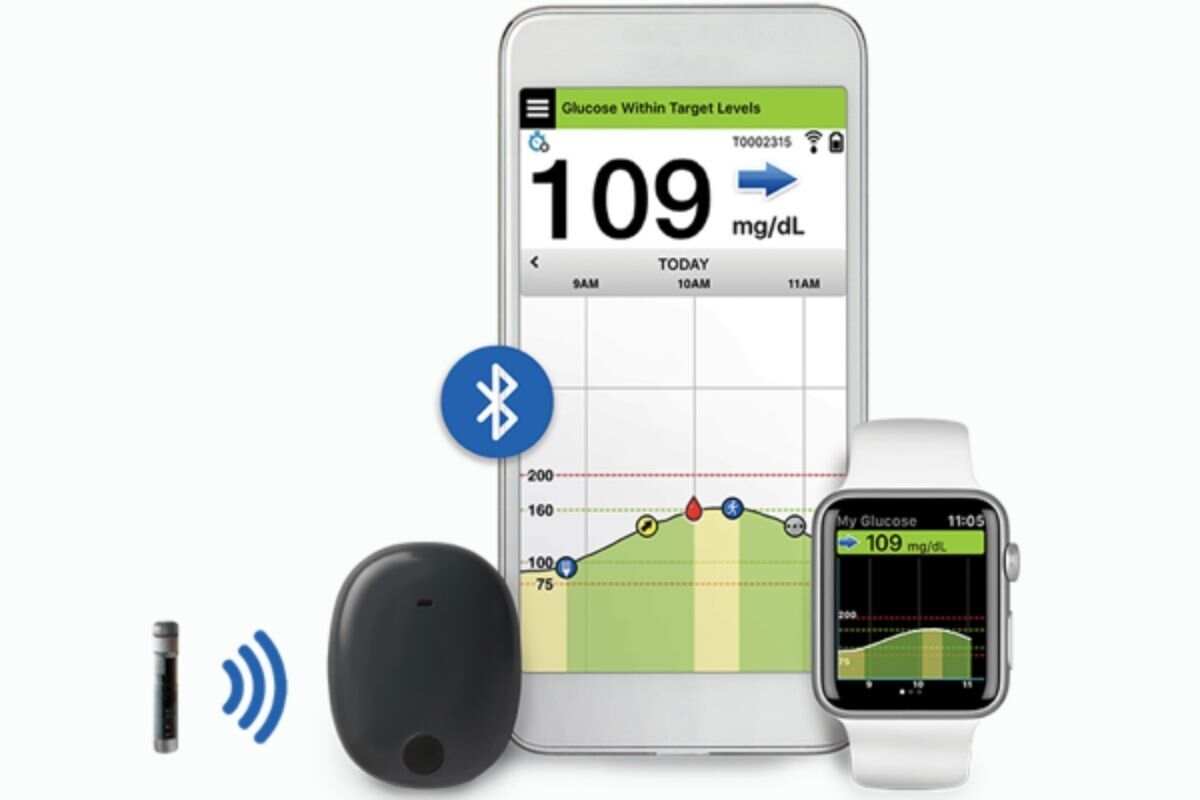
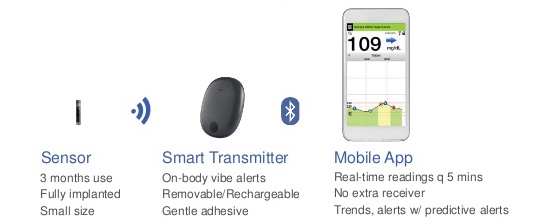
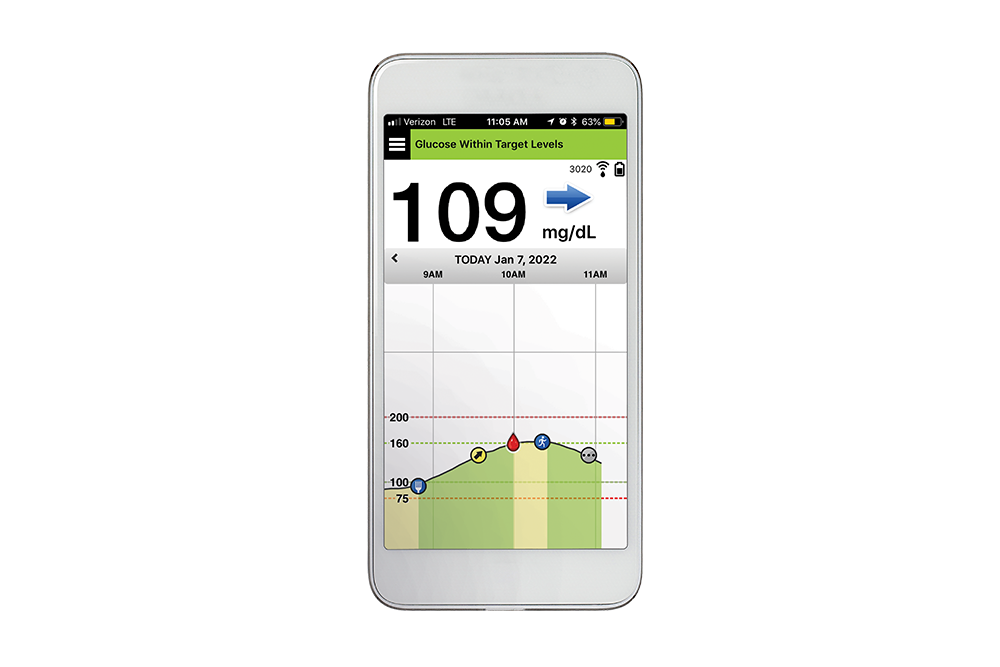
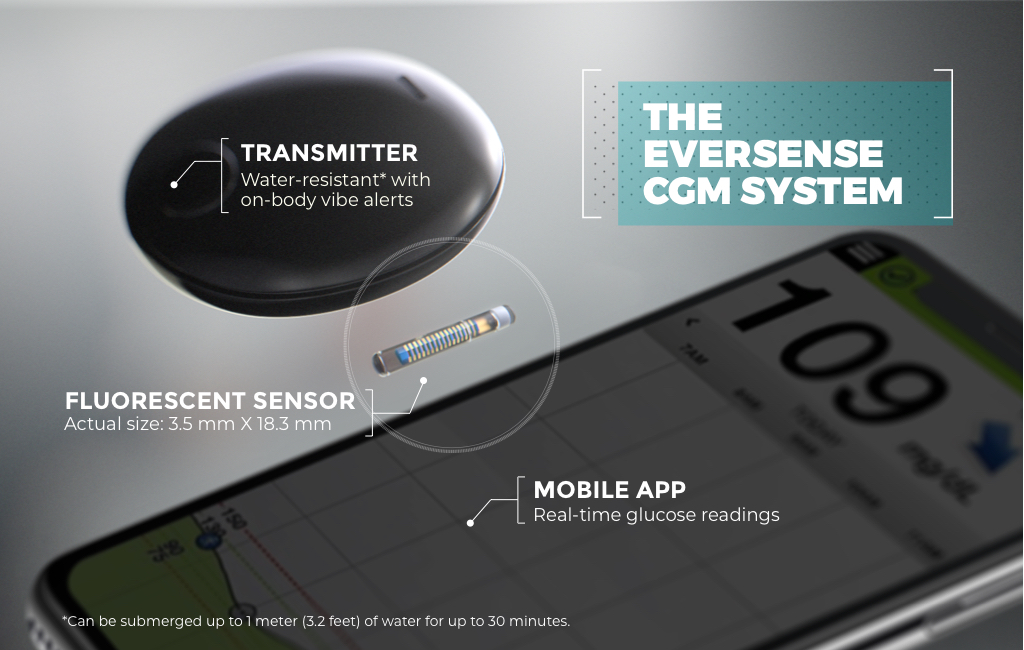
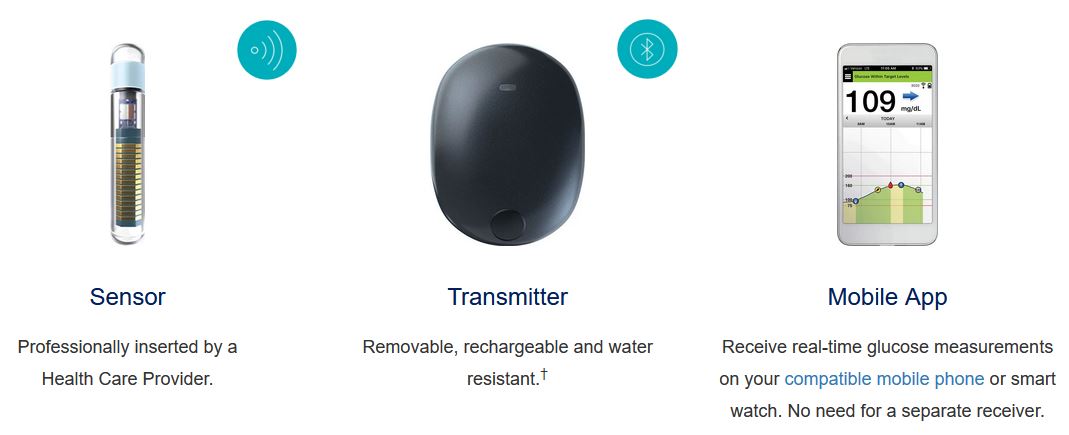
FDA approves Eversense E3 6-month continuous glucose monitor that requires fewer fingerstick blood glucose measurements - NotebookCheck.net News

Review of the Long-Term Implantable Senseonics Continuous Glucose Monitoring System and Other Continuous Glucose Monitoring Systems - Jeffrey I. Joseph, 2021